Monday, April 18, 2011
Wednesday, April 6, 2011
walking project-period 2
 Today we continued our walking experiment by walking to a location and finding the distance. We have to find out how many steps are taken in 10 seconds. We recorded each persons step and added them and multiplied by 6 and divides by 4. Our formula we need to follow was- EE was our walker and she walked an average of 27.5 steps in 10 seconds. We found out that she took 135 steps in one minute. We divided it by 60 and got 2.25 steps per second. We later realized how we messes up our experiment and had to start over. This time, we took the measuring tape and measured 50 feet. She walked the distance of that, which also failed. Mr. Finley explained to us to find the uncertainty of our steps. That is something we will continue tomorrow. Tomorrow we will also review the numbers that we found today and use uncertainty to receive a better answer for the number of steps S.D
Today we continued our walking experiment by walking to a location and finding the distance. We have to find out how many steps are taken in 10 seconds. We recorded each persons step and added them and multiplied by 6 and divides by 4. Our formula we need to follow was- EE was our walker and she walked an average of 27.5 steps in 10 seconds. We found out that she took 135 steps in one minute. We divided it by 60 and got 2.25 steps per second. We later realized how we messes up our experiment and had to start over. This time, we took the measuring tape and measured 50 feet. She walked the distance of that, which also failed. Mr. Finley explained to us to find the uncertainty of our steps. That is something we will continue tomorrow. Tomorrow we will also review the numbers that we found today and use uncertainty to receive a better answer for the number of steps S.D
Tuesday, April 5, 2011
Walking Lab
Sunday, April 3, 2011
March 31 #4
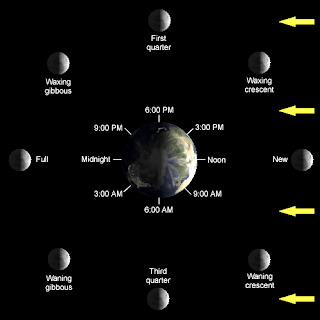
Today we had a substitute and he handed out Lunar Lab packets for us to do. We used a lunar simulation to answer the questions in the packet. Most of the questions were about the phases of the moon and how an observer on Earth would see the moon. We had to work in pairs to work on the packet but we didn't have to finish it. The substitute said we would have time to work on it the next day.
April 1st

Today, we spent the whole class finishing our Lunar Lab that we started the other day.
Tuesday, March 22, 2011
March 22nd, 2011 - Seasons and Sunlight

Monday, March 14, 2011
3/14 Phet Simulation

Saturday, March 12, 2011
Today is March 11, 2011
The first thing we did today was go over the homework.
We added in arrows to the dot diagram in between the dot because we realized that regular dot diagrams are way too primitive. The arrows show direction, and speed (the longer it is, the faster it goes)
The ball leaves your hand moving rather fast, but it slows down. This is made very clear by the ball stopping and hovering at the top of its ascent .
In C:There is only one dot in the diagram because at the top of its path, the ball stops and hovers for a very short time. This means it is not moving, and therefore there is only one dot.
Here is a video showing what would happen in this experiment. It will help explain the things discussed
Then Mr.Finley showed us an example. He pushed a dictionary across the table. The book left his hand, then moved across the table, slowied down, and finally stopped
Then we made a force diagram, and explain why this happens.
My group said that Mr. Finley exerted a horizontal force, but once it left his hand,
Monday, March 7, 2011
March 7th, 2011
AL had labeled her force diagram like this (on the top) force exerted from the Earth on Car, then the dot was labeled car, and on the bottom it was the force exerted from the road to on the car. But she had mixed the two up, the force from the Earth was on the bottom, and the road on the top, so JP fixed them.
JF had labeled hers as so; on the top, it was the force from the string and the middle was the picture with the frame and the bottom was the force on the earth
The arrows on the picture frame was very short because the picture frame isn't exerting as much force as the car.
Then Finley showed us a "video" on PHET that was a man pushing a box on the ice and we had to make a force diagram on the "video"
EC and I said that if force is exerted on the box, then the box is going to continue traveling for awhile, but before the earth's force is going to slow the box down.
JG (Fourth Time)
Thursday, March 3, 2011
3/3/2011 Period 2
Question 1 had a scenario about a jacket on a hook.
A said: what are the objects with which the jacket interacts? What object is our system?
When we discussed this in class we came up with the jacket touching the hook, and that the jacket was the point of interest and the system.
B said: Represent the situation with a force diagram.
The force diagram looked a little like this. The hook was pushing the jacket up, and the earth was pulling the jacket down. The arrows have to be equal because if the hook exerted more force than the earth the jacket would keep rising, if the earth was exerting more force than it would pull the jacket down to the ground.
Earth
C said: How would the force diagram change if you put a heavy book in your pocket.
If you put a book in your pocket than the diagram would look more like this. The earth would be exerting more force downward and the hook would be exerting more force upward. The jacket would still be in the s
 ame spot because the forces are still equal.
ame spot because the forces are still equal.Hook
0N
Question 2 was about 2 people carrying a suitcase.
You had to make a # sentence and a force diagram for A. The # sentence i used was +20 + -20 = 0, and this was my force diagram.

People
C asked how you decided which side you decided was to be positive and which negative. The positive side goes up because the positive side is always represented by going up or right and the negative side is always represented by left or down.
We didn't do anything else in class to today, but we do have 4 Q's for HW.
JB Post #3
Wednesday, March 2, 2011
New Unit - Astronomy
We started off class with a demonstration of holding a bowling ball in one hand and a ping pong ball in the other. Then we observed what happened.
- You have to push up to hold each ball
- There is a different push on both sides because of the weight difference
We then discussed a new term, force, and that you are applying force to the ball to hold it.
Force - Interaction between 2 or more objects. (push, pull, etc.)
Next, we talked about what the previous class had said about this demonstration.
"The student had to have done work to the ball in order to hold it up." This is not true because you are applying force to the ball, not work. The ball has gravitational potential energy when it's being held, but nothing is doing work to it.
QuEsTiOnS
+What would happen is this was the only force exerting the bowling ball?-The only force is pushing up, so it would keep going up and not come down
+Are there any other objects exerting a force?
-The earth is pushing the ball down.

There is an equal amount of force on both sides so it is balanced.
Our number statement for this scenario would be: +7 + -7 = 0
This is because you are giving the ball energy when you hold it above the ground. The earth is pushing down on the ball, making it lose its energy.
During class today, someone mentioned something about the displacement of the ball. I didn't really understand that and why it was displacement. Could anyone tell me about it?
EE (3rd blog)
Thursday, February 17, 2011
February 17, 2011 #3

Today in class, we reviewed atoms and subatomic particles again.
- atoms are made of smaller particles.
- those particles are called subatomic particles.
- there are three different types of subatomic particles: protons, neutrons, and electrons
Wednesday, February 16, 2011
February 16, 2011

Ever group discussed with there tables about how they are going to separate these.
Tuesday, February 15, 2011
Friday 18, 2011
- Gauge
- Test Tube
- Test Tube Grabber
- 15-20ml of Sugar
- Saucer
- Saucer 2
- Matches
- Bunsin Burner
Before we did anything, as a class, we talked about the background information we got for our lab which was the homework from the night before. Some the the information was about John Dulton, atoms, particle motions, conservation of mass, decomposition, synthesis, states of matter, and sugar(C12H22O11).
Our experiment was to decompose 20ml of sugar. Our first step was to weigh all of the items that i listed above.
Our hypothesis was that sugar is made up of 2 things. Water and Carbon because sugar is C12H22O11. The C part is carbon and the H20 part is water. So our prediction was that if we were to evaporate the water by boiling the sugar, there should only be carbon left in the test tube. Also if conservation of mass were true, the test tube with sugar in it should weigh less than before the water was boiled out of it.
We were ready to experiment. First thing we did was to turn on the Bunsin Burner. We took a match and we put the flame over the bunsin burner, and it was on fire. Then we took the test tube with sugar in it, and used the test tube grabber to put it over the fire. In about a minute we already saw changes happening. At the bottom of the tube that was being heated the most, it seemed like the sugar was turning into a liquid. Moments later, the liquid turned into a yellow-ish color. The level of the sugar started rising to the top of the test tube as water was evaporating. It turned purple like black and there was water particles escaping and we trapped it with the saucer. As more water particles came, it turned back into water and dripped down. After we felt like there was no more water left, we turned off the bunsin burner and put the carbon filled test tube away.
Later in the day, we came back to see how the carbon was like. It was still black and hardened. We took paper towels, wrapped the test tube, and smashed it. The carbon came out looking like this.
That was it with our experiment, but now we have a whole lab report to write about this. We need background information, hypothesis, prediction, materials, experiment, analysis, and a conclusion. The lab report will be due sometime around next week. The test will be on Friday.
Y.E
PS I can't add an image because the "add picture" button was not working for my computer. If I were to add a picture, I would have shown a burning fire, and a glass with the least density on the top and the most dense on the bottom
MP
Thursday, February 10, 2011
Thursday February 10th
Monday, February 7, 2011
Feb 7, 2011
Friday, February 4, 2011

Thursday, February 3, 2011
WR
Tuesday, February 1, 2011
February 1, 2011 - Different Types of Heating
- Convection
- Conduction
- Radiation



Monday, January 31, 2011
January 31, 2011 - Reviewing Matter HW (review for quiz)

 The bar chart basically explains:
The bar chart basically explains:  Initial: Some thermal energy
Initial: Some thermal energyFriday, January 28, 2011
-MM
Tuesday, January 25, 2011
MA 1-25
First, we did a practice problem for density: a soup can, with radius=2cm, h=5cm, and the mass=300g. We had to find the density. In order to, Finley gave us a cool pneumonic device to help us rememebr formulas for circles and cylinders: for a circle, it's 3.14r(squared), and since a cylinder is really a bunch of circles on top of each other, you multiply that equation by the height, h. You get 3.14r(squared)h. The density was 4.78 grams per cm.3
We also went over the homework. It would be hard to put up all the answers here, but i'll put some of the conclusion questions:
2. An object with a density of 0.67 kg/L would float 2/3 underwater.
3. A floating object has an upward force that is equal to the downward weight.
4. What would happen to an ice cube if it was dropped into a glass of 100% ethanol (density= 0.789 kg/L)? Would it be pushed up more or less? Why do you think this?
It would be pushed up more and down less. It would be because, if you look at the data, you can find this rule: if the object is less dense than the liquid, it floats. Contrariwise, if it is denser than the liquid, it will sink more.
http://en.wikipedia.org/wiki/Density
that helped me understand some of it.
-MA
3rd
Monday, January 24, 2011
1-24-11 Density
Notes:
The amount of particles is the mass.
Unit Rates:
In order to compare, the unit rate must be the same.
mass: 1 unit of volume
mass
---- = density
volume
You have to make the unit to 1, because it is easier to compare to the volume that way. The goal is to compare two masses.
Next we went online to phet. We are supposed to answer the questions posted on the website for homework.
To get to phet, you google phet. Then you click on play with sims, then density, then run now.
The website is below:
http://phet.colorado.edu/en/simulation/density"
In class, we began this while working with a partner.
We came up with the formula that density = 100* the %
KK this is my 3rd blog
Friday, January 21, 2011
January 21,2011-- Denisty
-- The size of the object doesn't change the density. Like materials will have the same amount of density even if one object is bigger than another for example, a life-size gold Jenna statue versus a a solid gold ring has the same density
21.6 g : 8 cm3
 This is an example of the website we worked on where all the cubes had the same amount of density.
This is an example of the website we worked on where all the cubes had the same amount of density.JF 3rd
Thursday, January 20, 2011
Wednesday's packet review (1-19-11)
Overview of the packet:
5.1
examine a book on your group's table.
a. We thought the book (our book) resembled a rectangular prism
b. You can learn about it's length ( 25.5cm), width (20.5cm), surface area (522.75cm^2, 102cm^2, and 82cm^2), volume (2091cm^3), and depth/height (4cm).
c. Perimeter to SA is basically the distance around an object/shape to the unit per area inside the object/shape. Area to volume is basically the same thing but 2 dimensional to 3 dimensional.
d. the volume of the book is 2091cm^3 and we got it by the formula l * w * h = v.
e. yes, there is a formula to find the volume of a soda can (cylinder) by h * c * r^2 = v.
f. No, you can't use the same approach to find the volume of a water bottle because it is made up of multiple 3d shapes instead of one.
5.2
a. Volume to length is practically 3d to 1d because length only measures one line. V to A is, like I said earlier, 3d to 2d. we measure volume in cubic centimeters.
b. There is more than one way to measure the volume of the given object (ping pong ball, coin, a toy, and a dice). A few ways are to use an overflow bucket, use a formula with any variables needed, or a graduated cylinder.
c. Answers may vary
d. You can write down the result of each measurement by adding the uncertainty. Also, if it is compared together with two different units, then you can transform the units into one and then add the uncertainty.
e. answers may very
f. answers may very but your answers should either be the same or close enough to compare.
g. answers may very but uncertainty added to each result should make both the same or even closer.
5.3

It is important to have many ways to find the volume of an object to double check your results and to give you a better understanding of the experiment.
5.4 (if I get an answer wrong you can explain the correct answer below)
a. 0.016666 repeating hours are in a min. 60min = 1hr
b. 1L = 0.2642 gallons 3.785L + one gallon
c. 1,000g = 1kg 0.001 kg = 1 g
d. 1,000,000cm^3 = m^3 0.000001 m^3 = 1 cm^3
e. 1cm^3 = 1ml 1ml/^3 = 1cm
Sorry that I couldn't post this yesterday. Comment if you have anything to say or anything to add!
BB (3rd Blog)
Density- P2

Monday, January 10, 2011
Burning Paper On January 11, 2011
Friday, January 7, 2011
First we continued to go over yesterday's homework.
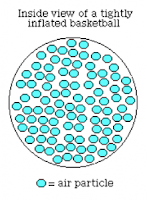
EC said that when you pump a basketball up, its not really
full because the particles inside are all moving around
GS continued her thought saying that air has a lot of empty space in it.
To test whether it is full or not, we could try to pump more air into the second picture.
Then we drew these two pictures. The first is before we pump more air in, and the second is after we pump more air in.

Thursday, January 6, 2011
1/6
Wednesday, January 5, 2011
January 5th 2011
My group came up with this hypothesis.
- Particles move faster with air. No air particle get in the way
Then the whole class joined together for a group discussion.
Davis said: Without the air there is more room and less resistance so it leaves the paper quicker. And it turns out it was a lot of group's hypothesises. Just different wordings.
Finley then said that that alcohol is made up of parts, like particles. Any thing that makes stuff up. Particles want to move, they don't want to move. They're naturally in motion.
Finley brought up a group and a single person. The group was blocking the single person from doing what he needed to do. It made it harder for the single person to move through the group.
We proved that the alcohol dried faster in the vacuum.
Finley brought another group up and put a chair and a container of ammonia with a group of people around it. It was a group of about 8-10 people. 4-5 girls 4-5 guys. They were instructed to raise their hand when they noticed something. 3-4 guys raised their hands.
Afterwords, we wrote down this;
- Hands rose very randomly
- In general, spread from middle outwards. (Not a perfect pattern)
- Shorter people got it first.
- JF & KK never detected the smell.
- Papi detected it 3rd.
The particles were explored farther;
- they move freely
- they move in a random manner
- they move in a direction.
- moves at random speeds.
Then we revisited the homework question:
If your mom is making pizza on the first floor and your on the second floor, how do you know what she's making for dinner?
A classmate said that you know what she is cooking because the particles in the food makes the scent.
Class ended soon after that. The homework is posted on Finley website.
JG
Monday, January 3, 2011
Monday January 3,2010- Observations and Mechanisms






-where the liquid hit the paper, the paper turned a darker shade
-the liquid smells like rubbing alcohol
-the paper gets wrinkled where the liquid touches the paper
-the liquid is disappearing
-the larger the spot of liquid, the more time it takes to disappear
-when the liquid was on the colored lines of the notebook paper, the lines blurred
After that, Mr. Finley told us that so far this year, we have been making explanations about the things that we have experimented. Explanations -------> why things happen. He said explained that we were now going to make mechanisms. Mechanisms -------> how things happen. Mr. Finley explained that mechanisms can be crazy ideas, but they have to be testable.
He started us of with an example mechanism for the liquid on paper. Ex. The table soaked up the liquid(the liquid went inside the table). We then started making mechanisms of the liquid on paper at our groups.These are the class's mechanisms
1. It went inside the paper
2. The air took it
3. Some other organism took it
4. When people smelled it the liquid on the paper for observations, it went up their noses
Lastly, we began to test our mechanisms. We made a chart with three columns. Th first column was the mechanism column, second was the test column, and the last column was the prediction column, when you think you are right. We only had time to fill out the chart for the example mechanism.
mechanism column: the table soaked the liquid up
test column: take the table away and repeat
prediction: the paper should stay wet
Before we could actually test the mechanism, the bell rung.
Some advice if people are getting mixed up with explanations and mechanisms. Think of them like this:
explanations=why
mechanism=how
There are some pictures of liquid on paper on the top of the of my post taken at 20 minute intervals.
CS

